BioHub 2D Disposable Bioprocess Bags
Deskripsi Produk
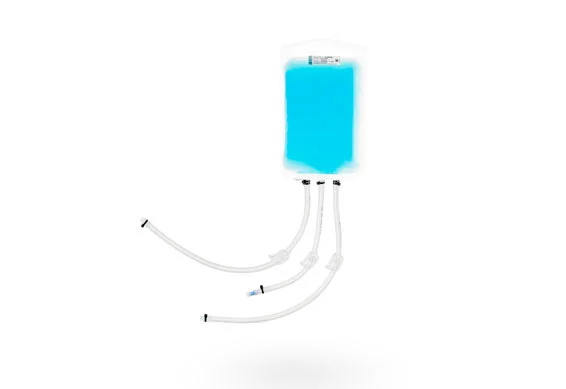
BioHub 2D Disposable Bioprocess Bags
BioHub® 2d single use system bioprocessing bags are designed for bioprocess needs. As a 2D biocontainer, it is suitable for liquid mixing, storage, transport, and cryopreservation.
Features of BioHub 2D Disposable Bioprocess Bags
100% integrity test of finished products after assembly;
Capacity: 110%;
Liquid recovery rate: 99.9%;
Traceability;
A flexible choice of film options;
Similar materials of construction, extremely low level of extractables and leachables;
High barrier property, optimizing the stability of products in the bag;
High transparency, easy to observe the status of products in the bag;
Animal-derived component-free (ADCF) materials, ensuring safety.
Customization.
Quality Control of BioHub 2D Disposable Bioprocess Bags
In controlled environments (ISO Class 7 environments).
Following ISO 9001:2015 certified quality system.
Animal-derived component-free (ADCF).
Meets USP , USP Class VI, USP , USP, USP , ISO11737-1, ISO11737-2, and ISO11137 for liquid contact materials.
Document support: Shipped with COQ or COA.
Sterilization: 25–40 KGy of gamma.
Shelf life: 2 years.
Packaging: Double bagged.
Specifications of BioHub® 2D Disposable Bioprocess Bags
BioHub® 2D storage bag adopts ship shape outlet, and the port size includes 1/8 ", 1/4" and 3/8 ". This 2D biocontainer can be connected to sili-cone tubing and Thermoplastic tubing to meet various liquid management requirements.
2D Storage Bags and Matching Holder Series
Trays for 2D storage bags: Plastic trays
Trays for 2D storage bags: Stainless steel trays
Cryopreservation cases for 2D storage bags
BioHub® Film
The films of BioHub® disposable bioprocess bags are from Renolit----the world's top film supplier. The biopharmaceutical-dedicated film is manufactured by the advanced multi-layer co-extruded technology, adequate certificates of material quality and validation documents are provided. Its physiochemical intensity, gamma irradiation tolerance, extractable level, and biological safety all meet the strictest standard of biopharmaceutical industry.
Features
Similar materials of construction, extremely low level of extractables and leachables;
High barrier property, optimizing the stability of products in the bag;
High transparency, easy to observe the status of products in the bag;
Animal-derived component-free (ADCF) materials, ensuring safety.
Film features
Biocompatibility (irradiation dose > 45 kGy)
Quality assurance system
The Bio-Link single-use product manufacturing factory has been certified by ISO9001:2015 and ISO13485:2016.

