Peripheral Stent System
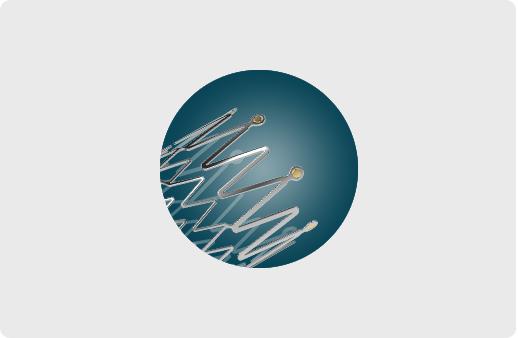
Product description
OVERVIEW
Peripheral Stent System
The ZENFLEX® Peripheral Stent System is designed to deliver a self-expanding stent to the iliac artery, superficial femoral arteries and/or proximal popliteal arteries via a 6F delivery system.
PRODUCT FEATURES
UNIQUE SPIRAL DESIGN
Excellent flexibility
Superior vascular compliance
EXCELLENT VASCULAR COMPLIANCE
Every 2mm length contains 7 connecting bridges
MICROMESH DESIGN
High radial strength and good fracture resistance
Maximizes vessel patency by decreasing tissue prolapse
GOLD/TANTALUM MARKER
Each end of the pvd stent embedded 6 micro markers
Accurately mark stent ends out and enhances visibility
CONTROLLED RELEASE SYSTEM
One-handed stent deployment
The initial precise positioning followed by well matching quick release
Ergonomic handle with anti-skid tuning dial combines comfortable hand feel and placement accuracy
Compatible with 0.035"guidewire
ORDERING INFORMATION
Usable Length Usable Length Stent Length Stent Diameter Usable Length Sheath
(800mm) (1250mm) (mm) (mm) (mm)
ZX04020080B ZX04020125B 20 4 800/1250 6F
ZX04030080B ZX04030125B 30 4 800/1250 6F
ZX04040080B ZX04040125B 40 4 800/1250 6F
ZX04060080B ZX04060125B 60 4 800/1250 6F
ZX04080080B ZX04080125B 80 4 800/1250 6F
ZX04100080B ZX04100125B 100 4 800/1250 6F
ZX05020080B ZX05020125B 20 5 800/1250 6F
ZX05030080B ZX05030125B 30 5 800/1250 6F
ZX05040080B ZX05040125B 40 5 800/1250 6F
ZX05060080B ZX05060125B 60 5 800/1250 6F
ZX05080080B ZX05080125B 80 5 800/1250 6F
ZX05100080B ZX05100125B 100 5 800/1250 6F
ZX05120080B ZX05120125B 120 5 800/1250 6F
ZX05150080B ZX05150125B 150 5 800/1250 6F
ZX06020080B ZX06020125B 20 6 800/1250 6F
ZX06030080B ZX06030125B 30 6 800/1250 6F
ZX06040080B ZX06040125B 40 6 800/1250 6F
ZX06060080B ZX06060125B 60 6 800/1250 6F
ZX06080080B ZX06080125B 80 6 800/1250 6F
ZX06100080B ZX06100125B 100 6 800/1250 6F
ZX06120080B ZX06120125B 120 6 800/1250 6F
ZX06150080B ZX06150125B 150 6 800/1250 6F
ZX07020080B ZX07020125B 20 7 800/1250 6F
ZX07030080B ZX07030125B 30 7 800/1250 6F
ZX07040080B ZX07040125B 40 7 800/1250 6F
ZX07060080B ZX07060125B 60 7 800/1250 6F
ZX07080080B ZX07080125B 80 7 800/1250 6F
ZX07100080B ZX07100125B 100 7 800/1250 6F
ZX07120080B ZX07120125B 120 7 800/1250 6F
ZX07150080B ZX07150125B 150 7 800/1250 6F
ZX08020080B ZX08020125B 20 8 800/1250 6F
ZX08030080B ZX08030125B 30 8 800/1250 6F
ZX08040080B ZX08040125B 40 8 800/1250 6F
ZX08060080B ZX08060125B 60 8 800/1250 6F
ZX08080080B ZX08080125B 80 8 800/1250 6F
ZX08100080B ZX08100125B 100 8 800/1250 6F
ZX08120080B ZX08120125B 120 8 800/1250 6F
ZX08150080B ZX08150125B 150 8 800/1250 6F
ZX09020080B ZX09020125B 20 9 800/1250 6F
ZX09030080B ZX09030125B 30 9 800/1250 6F
ZX09040080B ZX09040125B 40 9 800/1250 6F
ZX09060080B ZX09060125B 60 9 800/1250 6F
ZX09080080B ZX09080125B 80 9 800/1250 6F
ZX09100080B ZX09100125B 100 9 800/1250 6F
ZX09120080B ZX09120125B 120 9 800/1250 6F
ZX09150080B ZX09150125B 150 9 800/1250 6F
INDICATIONS
Peripheral Stent System
The ZENFLEX® Peripheral Stent System is indicated for improving luminal diameter in the treatment of patients with de novo or restenotic native lesion(s) of the iliac artery, superficial femoral artery and/or proximal popliteal artery with total lesion length up to 150 mm and with a reference vessel diameter ranging from 3 mm to 8 mm.
We are one of leading neurovascular medical device companies in the peripheral and neurovascular interventional medical device market in China. As a fully integrated medical device company, Tonbridge medical has strong R&D and manufacturing capabilities, multiple specialized technology platforms, and proven commercialization capabilities. Led by our talented management team, we strive to provide best medical services and cutting-edge technical solutions to physicians and patients in China and around the world.

